
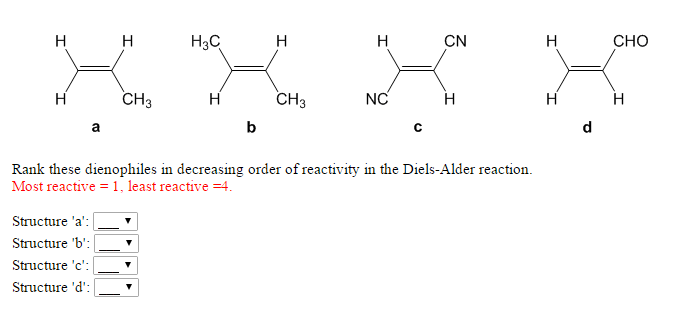
The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles this variant is known as the hetero-Diels–Alder reaction. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. It was first described by Otto Diels and Kurt Alder in 1928. More specifically, it is classified as a thermally-allowed cycloaddition with Woodward–Hoffmann symbol. It is the prototypical example of a pericyclic reaction with a concerted mechanism. It would be too much for today, so you can check this post.In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. If the diene is also unsymmetrical, then you need to consider the regiochemistry of the Diels-Alder as well. If the cyclic diene is reacted with a mono-substituted dienophile then the product contains one stereogenic center and the endo and exo products are formed as two enantiomers:Īnd any combination of an endo and exo product represents a pair of diastereomers: You can read more details such as the transition state and the endo-exo definition when acyclic dienes are reacted here. In general, endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. In the endo product, the substituents of the dienophile are pointing towards the larger bridge, while in the exo isomer, they are pointing away from the larger bridge: The main concept here is the endo-exo isomerism. If the diene is cyclic as well, then bicyclic compounds are formed. This indicates that Diels-Alder is a stereospecific reaction: Some of the commonly used dienophiles are shown below:Ī few more points about the stereochemistry of the Diels-Alder reaction depending on the structure of the diene and the dienophile.įirst, remember that if there are two groups on the dienophile, the product will have them cis or trans exactly as they initially appear in the dienophile. Therefore, electron-donating groups on the diene increase its reactivity, while electron-withdrawing groups on the dienophile lower the LUMO energy level, thus support this electron flow as well.Īs we just mentioned above, electron-withdrawing groups increase the reactivity of the dienophile. Even though it is a concerted mechanism, one can imagine the electron flow being initiated from the highest occupied molecular orbitals (HOMO) of the diene and goes to the lowest occupied molecular orbitals (LUMO) of the dienophile. This is a result of the HOMO-LUMO interaction in the Diels-Alder reaction.

Remember that electron-donating groups increase the reactivity of the diene: The second factor is the electronic effect. Cyclic dienes produce bridged bicyclic compounds: These conformations are two extremes as the locked trans dienes do not react in the Diels-Alder while the cyclic cis dienes are so reactive that they may react with themselves.įor example, cyclopentadiene dimerizes because one molecule acts as the diene and the other as the dienophile.
REACTIVITY OF DIENOPHILES FREE
Cyclic dienes, on the other hand, are locked whether they are cis or trans since there is no free rotation about the sigma bond:
However, these are conformations since they interconvert through a rotation abound the sigma bond between the two double bonds and we call them s- cis and s- trans:Īcyclic dienes usually prefer the s- trans conformation. Conjugated dienes can also be in cis and trans geometry. We are not talking about the traditional cis and trans isomerism of the alkenes. The first thing to remember here is that it can only react in the cis conformation. First, a reminder that the Diels-Alder reaction is a type of a pericyclic reaction between a conjugated diene (two double bonds) and a dienophile (an alkene with an electron-withdrawing group). In this post, we will discuss the reactivity and specifics of the diene and the dienophile in the Diels-Alder reaction.


 0 kommentar(er)
0 kommentar(er)
